Blog
Instrument Reprocessing without the “Magic Window”

Three Key Insights Every New Dental Graduate Should Know
Students at dental, dental hygiene, and dental assisting schools have very full plates. Whether in the classroom or in-clinic, there is a lot to learn in a relatively short amount of time. But one area where dental students sometimes get a break is in instrument reprocessing. At many programs – particularly dental programs and some hygiene programs – instrument reprocessing is not handled by students but rather staff behind the “Magic Window” where students return dirty or used instrumentation to be cleaned, disinfected, sterilized, and stored.
While the “Magic Window” is a great resource for students, without proper training young dental professionals may not fully understand the scope and importance of proper reprocessing and how it impacts the entire practice. It goes without saying that properly processed instruments are a must for infection prevention, but many dental students may not realize just how critical it is to get that process right from both a safety and compliance standpoint. There are several government agencies that oversee infection prevention and control in dentistry – including the CDC, OSHA, and state dental boards – and it can be challenging to achieve compliance with instrument reprocessing protocol.
Young dentists often don’t realize that while they might assign many of these responsibilities to their staff, they are ultimately responsible for ensuring their office is meeting all government agency standards. And because dental practices may not always get everything right, young hygienists, assistants, or other staff members need to have a good baseline understanding of the process to ensure they are properly trained on this critical function. This is also important when bringing on a new hire who may bring bad habits from a previous employer.
So, if you are a student or recent graduate (or just looking for a refresher on the topic), here are three key insights to know about instrument reprocessing as you begin your career:
- Instrument Mangement Systems Improve Efficiency and Safety
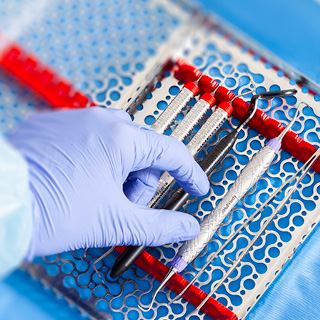 Instrument reprocessing takes time, and handling contaminated instruments is a safety risk for staff. Instrument Management Systems like the IMS™ Cassette address those challenges because they are designed to keep instruments organized and intact throughout the entire process, from cleaning to chairside. In fact, many dental, dental hygiene, and dental assisting schools use cassettes for this reason, so students may already be familiar with the concept even if they haven’t utilized them directly.
Instrument reprocessing takes time, and handling contaminated instruments is a safety risk for staff. Instrument Management Systems like the IMS™ Cassette address those challenges because they are designed to keep instruments organized and intact throughout the entire process, from cleaning to chairside. In fact, many dental, dental hygiene, and dental assisting schools use cassettes for this reason, so students may already be familiar with the concept even if they haven’t utilized them directly.
IMS™ Cassettes eliminate time-consuming tasks like instrument scrubbing and sorting, while making it easy to standardize procedural set-ups and train new staff. Those small time-savings throughout the day can add-up and lead to more time spent in the chair with patients.
Cassettes provide safety benefits as well because the handling of contaminated sharps is minimized since instruments stay secured throughout the entire reprocessing cycle, reducing the risk of injury or exposure to bloodborne pathogens.
Although Instrument Management Systems are not required for dental practices, they provide better efficiency and improved safety, which is ultimately a win-win for all involved in instrument reprocessing.
- Instrument Reprocessing Requires Specific PPE
 While a lot of attention is given to PPE used in the operatory, it’s just as important to get it right for instrument reprocessing. Proper PPE, as defined in the CDC’s Guidelines for Infection Control in Dental Health-Care Settings published in 2003, must always be worn when reprocessing contaminated patient equipment.
While a lot of attention is given to PPE used in the operatory, it’s just as important to get it right for instrument reprocessing. Proper PPE, as defined in the CDC’s Guidelines for Infection Control in Dental Health-Care Settings published in 2003, must always be worn when reprocessing contaminated patient equipment.
Always use utility gloves when handling instruments and cassettes following patient treatment. Using patient exam gloves instead of puncture and chemical resistant utility gloves puts dental professionals at risk for chemical exposure and sharps injuries. Utility gloves should not be bulky and should enable tactical sensitivity. Contaminated utility gloves should only be worn on the dirty side of the sterilization area and should be disinfected according to the manufacturer’s IFU. Gloves are not required when removing wrapped cassettes or packs from the sterilizer once the sterilization cycle has been completed.
In their latest Interim Guidance for dental settings during the COVID-19 pandemic, the CDC provides additional direction for general PPE usage while reconfirming that dental health care professionals should continue to follow the recommendations for instrument reprocessing laid out in their 2003 guidelines.
- Instrument Reprocessing Is a Patient Showcase Opportunity
More than ever, patients are looking for reassurance that dental practices are emphasizing infection prevention and control, and instrument reprocessing can be a good showcase to help differentiate a practice.
As an example, some practices feature an open, centralized steri-center to give patients an opportunity to view reprocessing and see the steps being performed. Additionally, In the past, one strategy was to give patients a tour of the steri-center, so they can see for themselves how the practice goes above-and-beyond. While this is not viable during the ongoing pandemic, there are other ways to demonstrate cleanliness, organization, and safety.
If your practice uses IMS™ Cassettes, you can open the cassette in front of the patient as a demonstration. A well-organized and nicely presented cassette can make a world of difference when compared to a tray of loosely organized instruments.
Additional Resources for Instrument Reprocessing
Instrument reprocessing is a big topic, and this only begins to scratch the surface. Use these resources to dive deeper into specific procedures, product solutions, and best practices:
- GreenLight Dental Compliance Center™ by HuFriedyGroup
- IMS™ Cassette Success Stories
- Webinar: Why Instrument Reprocessing Matters
- Request a Free IMS Consultation
All company and product names are trademarks of Hu-Friedy Mfg. Co., LLC, its affiliates or related companies, unless otherwise noted.

